Category: Technical Documents
Date: 2013-04-22
Click: 1954
Author: 佚名
Collection:
Bulk chemicals or "commodity chemicals" are a broad chemical category including polymers, petrochemicals, other derivatives and basic industrials, inorganic chemicals, and fertilizers. Typical growth rates for basic chemicals are about 0.5 to 0.7 times a country’s GDP and product prices are generally less than fifty cents per pound. Mixing and chemical reaction are closely related topics as chemicals need to be agitated in order to make contact and react with one another. Insufficient mixing and mass transfer could influence reaction rate and product distribution. In 1989, the cost of poor mixing was estimated at $1 billion to $10 billion in the U.S. chemical industry alone [3]. In one multinational chemical company, lost value due to poor mixing was estimated at $100 million per year in 1993 [3] Yield losses of 5% due to poor mixing are typical [3].

Figure 1. An industrial-size stirred reactor with multiple impellers and baffle
Examples of industrial applications of mixing in the chemical industry [3] include:
? Mixing miscible/dispersing immiscible reactants
? Dissolving gases (e.g., chlorination processes)
? Providing plug flow and controlled-reaction conditions in tubular reactors with low or high viscous fluids
? Dispersing liquids in extraction and washing processes
? Mixing gases in front of catalytic reactors (e.g., the production of styrene, nitric acid, maleic anhydride)
? Vaporizing liquids in front of oxidation reactors (e.g., xylene in phthalic anhydride plants)
? Co-current scrubbing acid process gas components
? Homogenizing process and product streams for representative sampling
? Neutralizing or pH adjustment/control of process streams with caustic or acid
The mixing issues of the following four chemical reactions plus a crystallization process, which a chemical engineer named Marco came across in a chemical plant, are vivid real life problems for mixing of bulk chemicals. These cases also serve as an overview to the subentries below [3]
Competitive-consecutive reaction. Bromination of an aromatic ring was the intended reaction, but due to overreaction, a second bromine was being added. This reduced selectivity could be caused by differences in micro-mixing and meso-mixing on scale-up. However, testing at two extreme stirring conditions did not show a difference in overbromination. A reversed addition of reactant to brominating reagent reveals overreaction. Therefore, insufficient mixing will result in lower selectivity due to competitive or consecutive side reactions.
Gas-liquid reaction. A hydrogenation reaction required 8 hours of reaction time, which was inherently slow according to the chemist. Upon careful inspection of the autoclave, it was revealed that surface reincorporation was not achieved due to high z/T ratio and full baffles. After Marco cut the baffles in order to create a vortex to the top of the pitched blade turbine, the reaction finished within half an hour. Reactor design is an important factor to improve the mixing for this type of reaction. Liquid viscosity determines the basic mixing mechanics and types of equipment to use in practice.
Solid-liquid reaction. An alkylation using powdered potassium carbonate to react with an organic acid in solution was scaled up to the pilot plant. The reaction was slower than expected and clogging happened at the bottom outlet of the reactor. Solid dissolving issues and inadequate mixing for the off-bottom suspension were solved by using a pitched blade turbine impeller and full baffles. The sensitivity of reaction rate to both particle size and impeller speed was readily established.

Figure 2. Example of a solid-liquid reaction within an agitated vessel.
Liquid-liquid reaction. A change of reaction vessel and impeller caused a drop in conversion rate and selectivity. Liquid-liquid complex reactions were considered the most difficult reaction scale-up mixing problem. Drop formation and coalescence change with scale, while location in a vessel has a very subtle change in fluids compositions. A quick “fix” is to increase impeller speed. In the meanwhile, overmixing could improve rate while reducing selectivity by exposing the product in droplet film to a higher concentration of reagent in the aqueous phase.
Reactive crystallization. Reactive crystallization presents critical mixing issues because mixing affects both the reaction and crystallization step. Marco’s group had always had difficulty with reactive crystallization scale-up due to:
1) The need to balance the requirements to achieve a growth-dominated process
2) Choose a mixing system fast enough to micro-mix effectively for the fast reaction
3) Ensure mixing is not too powerful that it will cause crystal fracture.
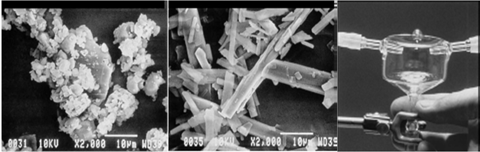
Figure 3. Equal surface area products obtained by milling larger crystals and by impinging jet crystallization (with improved product stability as well)
The above issues will be addressed in four subentries: reactor design, heterogeneous reactions, scale-up of reactors and crystallization. In summary, reaction type dictates the reactor design and scale-up of reactors. Mixing is very important in heterogeneous reactions to avoid mass transfer limitation and in crystallization processes to control crystal size and minimize impurity-uptake.





